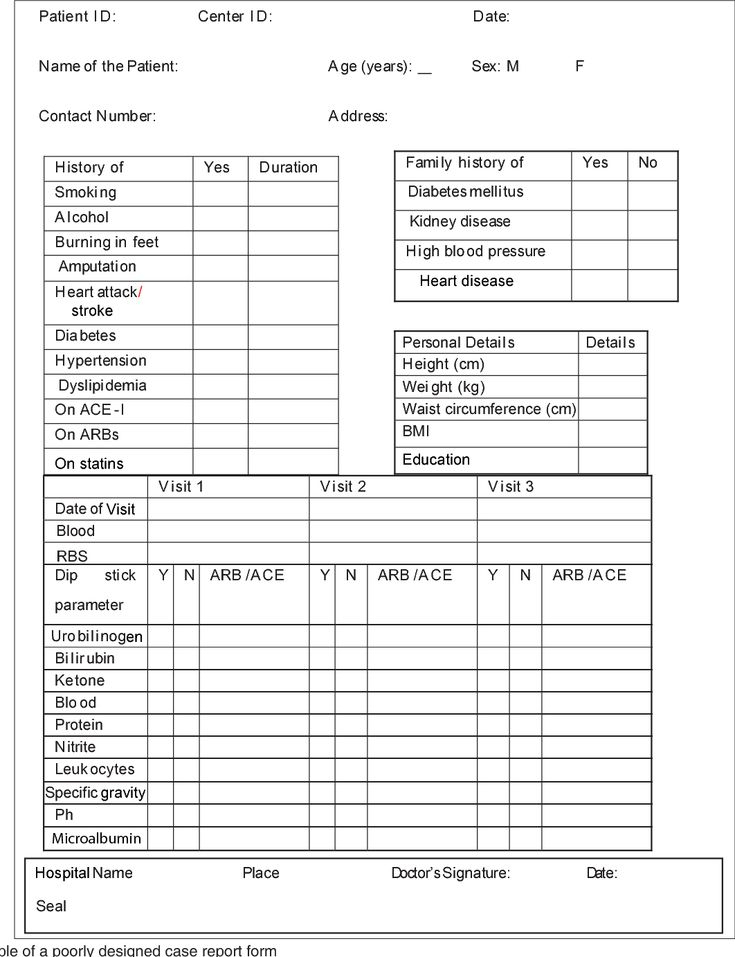
Clinical Trial Consent Form – Everyone should be able to make informed decisions regarding their healthcare. Medical treatments can be sensitive, so patients must be able to determine the risks that are known to be present as well as their own personal preferences, how they will be treated. In order to ensure that medical professionals are permitted to be able to treat their patients, they must receive what is known as informed consent.
A patient’s informed consent can be a legally binding requirement under which a patient is provided with detailed information about his or her physical health and the treatment recommended by the treating physician. After receiving this information patients must be able to give the physician their consent to treat prior to any form or treatment can be administered. Without the patient’s informed consent, a health care provider cannot offer treatments.
Decision Making Capacity
In some instances the patients aren’t equipped with the capabilities to fully understand the options for treatment and the risks/benefits associated with each one. In other situations patients might not be able to effectively explain their decisions to health workers. In these situations, the patient is said not to have adequate decision making capacity. The family member, or court appointed representative could then be able to provide informed consent instead.
Patients who are greatly influenced by their emotions, like anxiety or fear, for example are deemed not able to make decisions. Those who are unconscious clearly cannot make decisions on own. Therefore, outside parties must provide consent for treatment instead.
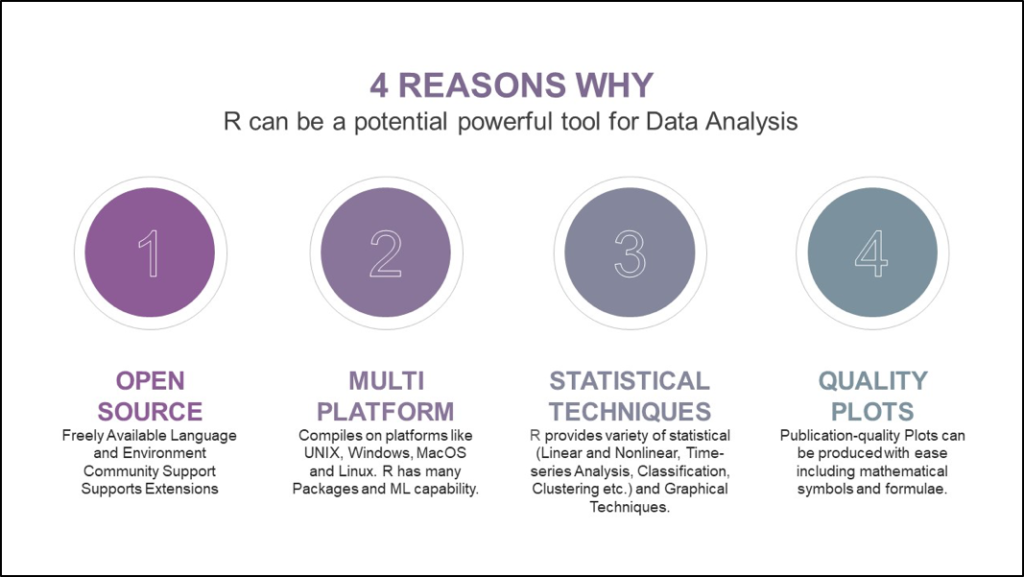
Items in an Clinical Trial Consent Form
There are certain elements that are generally included in informed consent forms:
The patient’s medical diagnosis/condition
The treatment that is recommended by the doctor in charge
The risks and benefits associated with this procedure
Alternative treatments are readily offered, as are their benefits and risks
The potential risks and rewards with accepting no treatment whatsoever
Not only must these items be detailed in documentation however, they must have a discussion with the patient. So, he is able to fully comprehend all the details of the scenario and will be able to get immediate answers to any concerns that might be arising.