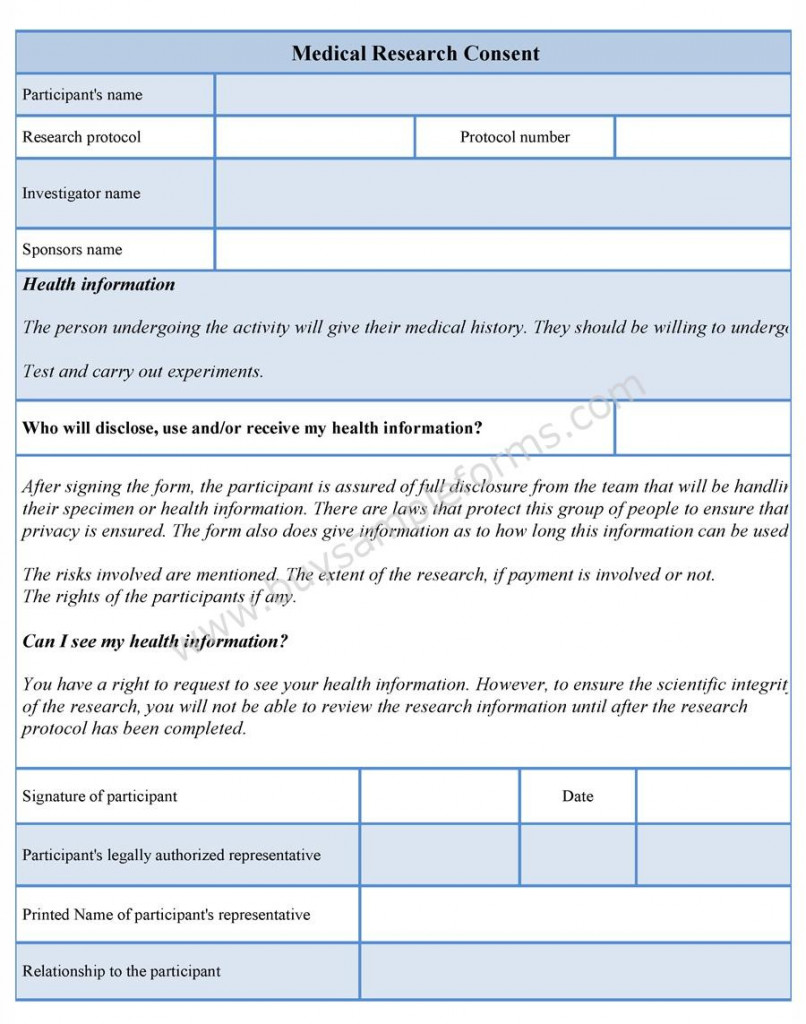
Consent Form For Clinical Research – Every person should be able to make informed decisions regarding their medical care. Medical procedures can be demanding, and therefore patients should be able decide in light of known risks as well as their own personal preferences, how they will be treated. In order to ensure that medical professionals are permitted to administer treatments to patients, they have to obtain the process of informed consent.
Informed consent constitutes a lawful condition where a patient is provided with detailed information about his or her physical condition and the treatment suggested by the physician who is acting as the patient’s physician. Once this information is received patients must give the doctor their consent to treat before any form of treatment is offered. Without the patient’s informed consent an health care professional cannot offer treatment.
Decision Making Capacity
In some instances the patients aren’t equipped with the capabilities to fully understand their treatment options , as well as the potential risks and benefits associated with each one. In other situations patients might not be able explain their decisions to health workers. In these situations the patient is considered not to possess the proper capacity for decision-making. An individual from the family or court-appointed representative, can take over informed consent.
Patients who are greatly influenced by their emotions – such as anxiety or fear, for instance are deemed lacking the ability to make decisions. Patients who are in the state of unconscious cannot take decisions on their independently, and other people are required to obtain consent instead.
Items in an Consent Form For Clinical Research
There are certain elements that are generally included in informed consent forms:
The diagnosis or medical condition of the patient.
The procedure recommended by the physician in charge
The risks and benefits associated with this treatment
Alternative treatments are readily available, as well as their benefits and risks
The risks and benefits associated with accepting no treatment at all
These details must not only be recorded in the patient’s medical records However, they should also have a discussion with the patient. This way, he or can fully comprehend what is happening and get straight answers to any questions that be arising.